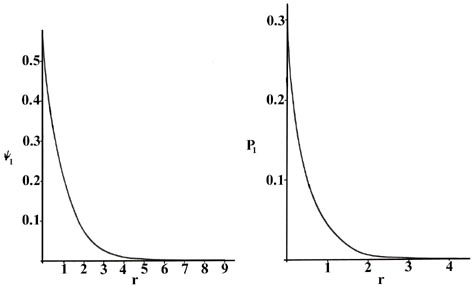
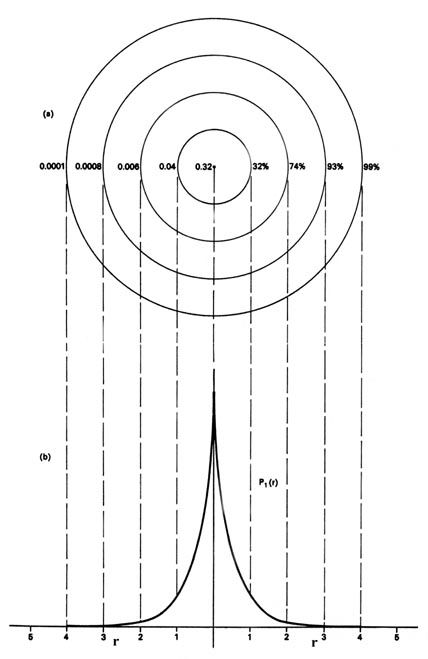
The radial distribution function is plotted in fig. The radial distribution function q1r for an h atom.

Hydrogen Radial Probabilities
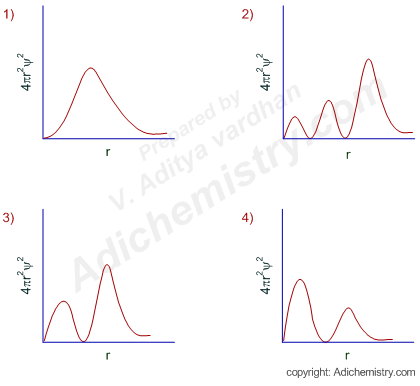
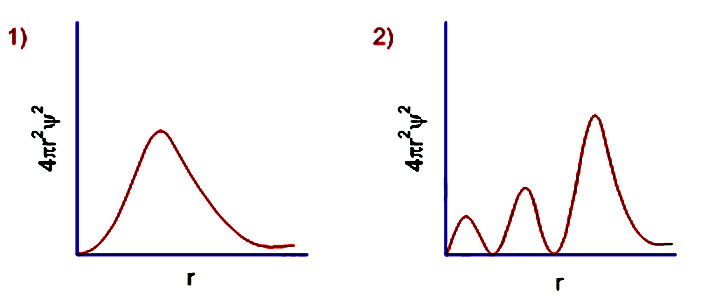
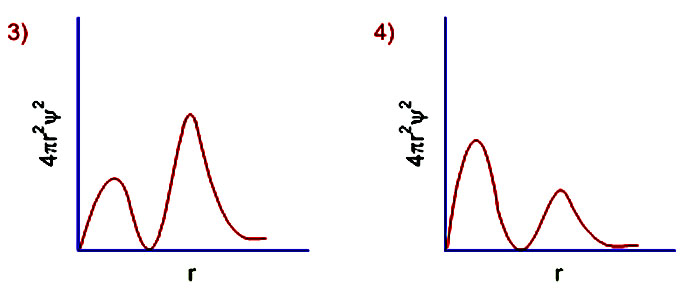
6 radial probability distribution curves are the plots of 4pr 2 ps 2 vs distance from the nucleus.

Radial probability distribution hydrogen atom. Radial probability distributions for the 1 s 2 s and 3 s orbitals of hydrogen. The radial distribution function is plotted in figure pageindex2 for the ground state of the hydrogen atom. 3 5 for the ground state of the hydrogen atom.
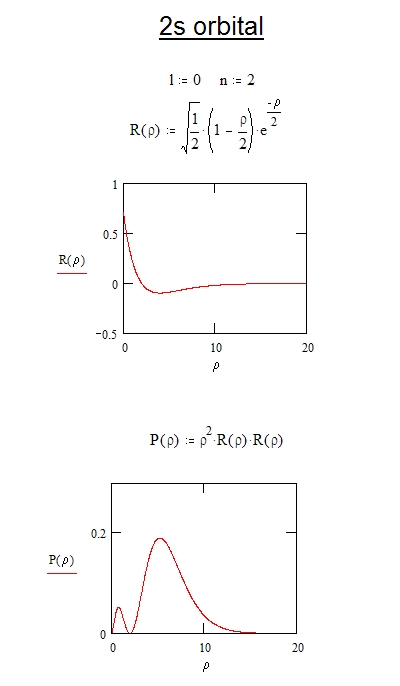
The eigenfunctions in spherical coordinates for the hydrogen atom are where and are the solutions to the radial and angular parts of the schrodinger equation respectively and and are the principal orbital and magnetic quantum numbers with allowed values and the are the spherical harmonics and the radial functions are where is the order associated laguerre polynomial and is the. The electron in a hydrogen atom is described by the schrodinger equation. Expectation value for radius.
The product 4pr 2 p n is given a special name the radial distribution function which we shall label q n r. Probability for a radial range. Index periodic table hydrogen concepts.
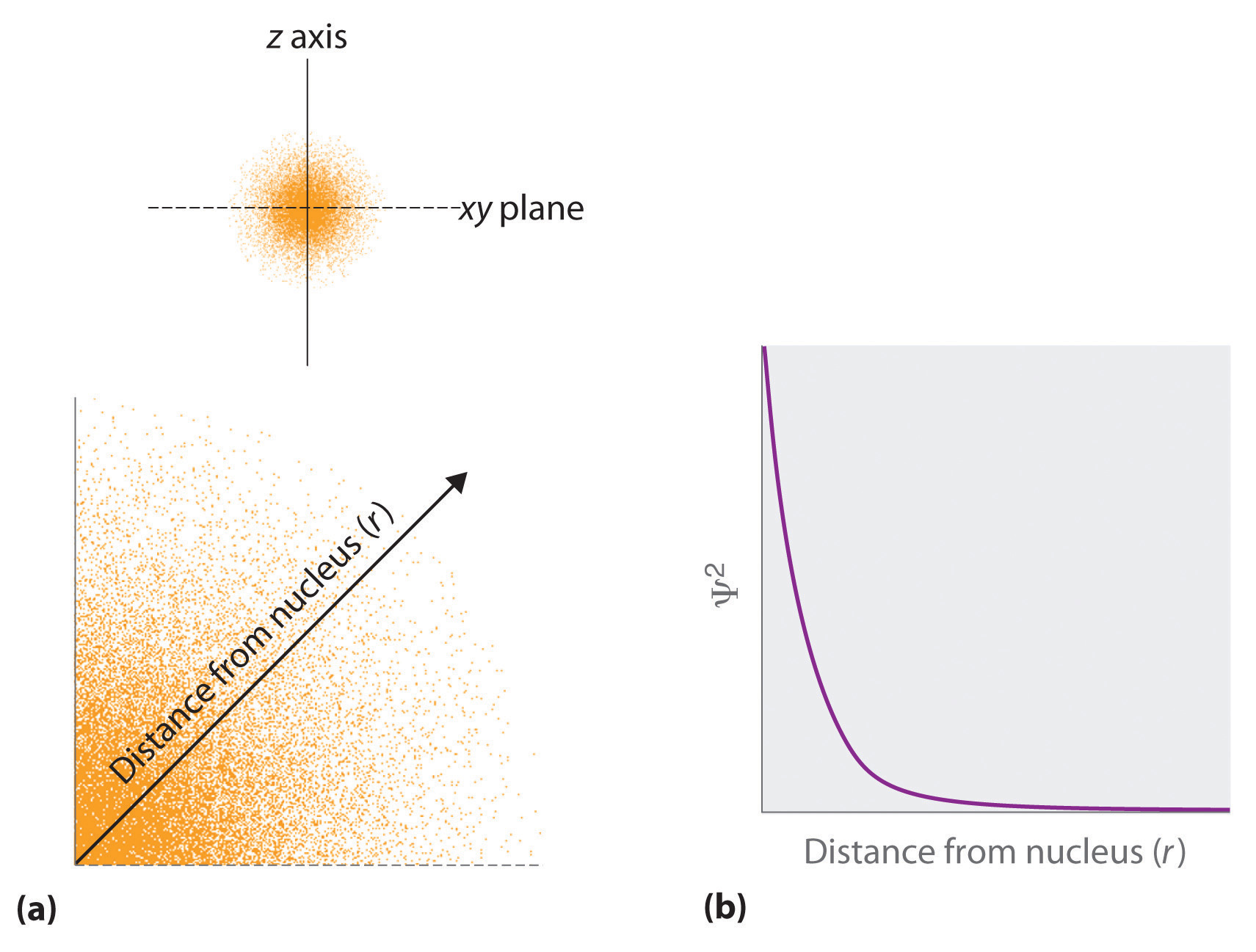
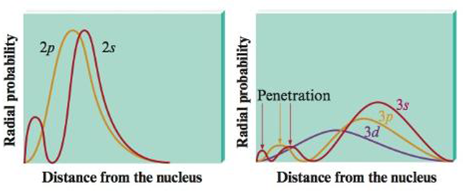
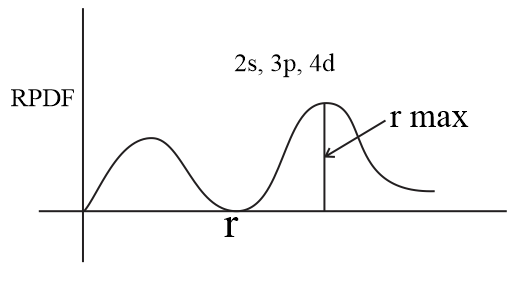
The value of this function at some value of r when multiplied by delta r gives the number of electronic charges within the thin shell of space lying between spheres of radius r and r. Radial behavior of ground state. The curve has number of maxima which is different for different orbitals.
These plots show the probability of finding the electron as a function of distance from the nucleus. As n increases the most likely distance at which to find the electron the highest peak moves farther from the nucleus. Hydrogen 1s radial probability click on the symbol for any state to show radial probability and distribution.
It is equal to the bohrs radius of 1st orbit in hydrogen atom. The time independent schrodinger equation in spherical polar coordinates can be solved by separation of variables in the form the radial and angular components are laguerre and legendre functions thus and respectivelyhere is the first bohr radius and are the integers in the ranges principal quantum number.

Hydrogen Radial Probabilities
Radial Probability Distribution Curves Atomic Orbitals

Radial Probability Distributions For The 1 Clutch Prep

Radial Probability Density Of The 3s And 3p Orbitals In Atomic Units Download Scientific Diagram

Radial Probability Distribution Of Hydrogen Atom In Ground State Youtube

Normalized Radial Probability Distributions For Hydrogen Atom Exploring Chemistry 3rd Edition

Hydrogen Radial Probabilities

Radial Probability Distribution Curves Quantum Mechanics Csir Net Gate Chemistry Iit Jee Jam Neet Youtube
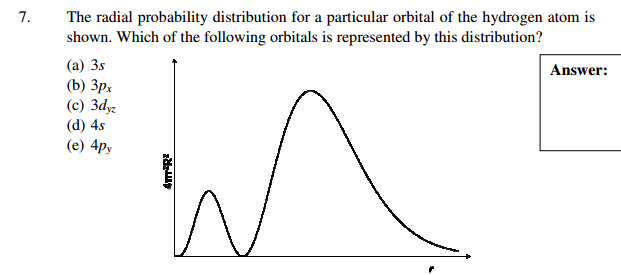
Solved 7 The Radial Probability Distribution For A Parti Chegg Com

Hydrogen Radial Probabilities
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com

Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom

Hydrogen Radial Probabilities

Physics 3740 Introduction To Relativity And Quantum Mechanics

Https Www Embibe Com Study Examples On Radial Probability Distribution Curve Concept
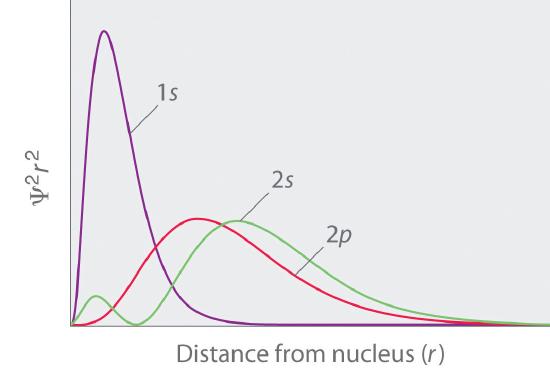
3 3 The Probability Distribution Of The Hydrogen Atom Chemistry Libretexts

Hydrogen Radial Probabilities
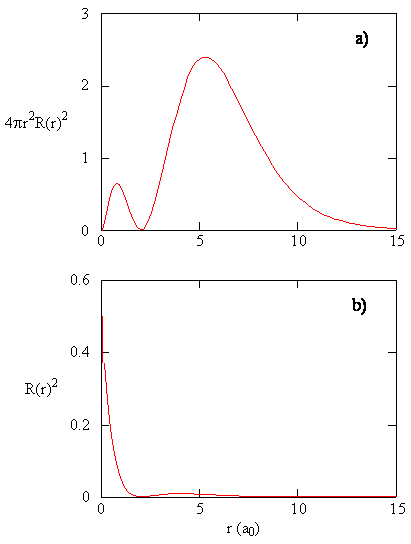
8 2 The Wavefunctions Chemistry Libretexts
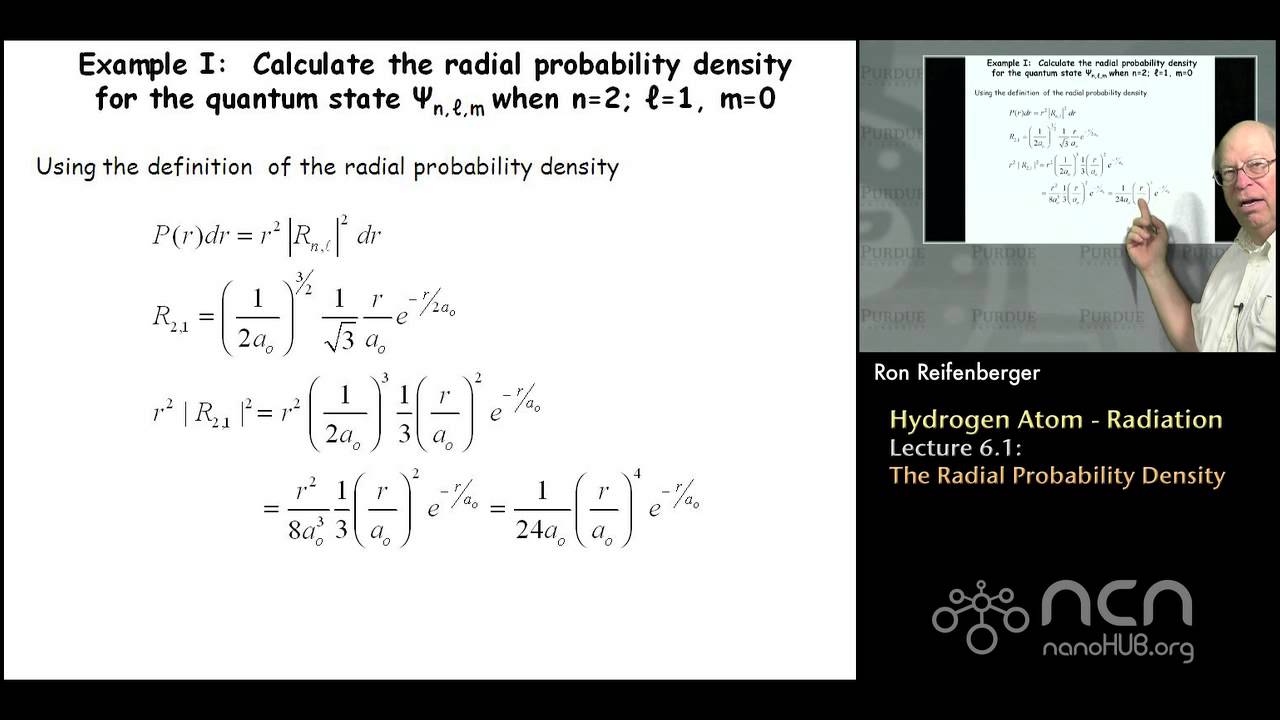
Purdue Phys 342 Modern Physics L6 1 Hydrogen Atom The Radial Probability Density Youtube
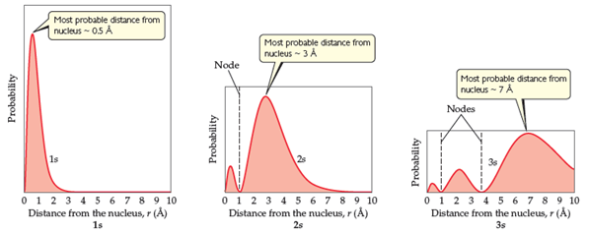
Solved Quantum Mechanics And Atomic Orbitals Sections A Wit Chegg Com

Structure Of A Hydrogen Atom English

Hydrogen Atom

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrghga0ooj Vhioszfatpprdyurcdmzptofnputdbx2rits4zr7 Usqp Cau
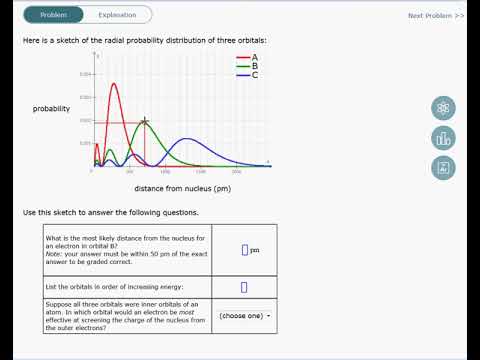
8 1a Interpreting The Radial Probability Distribution Of An Orbital Youtube
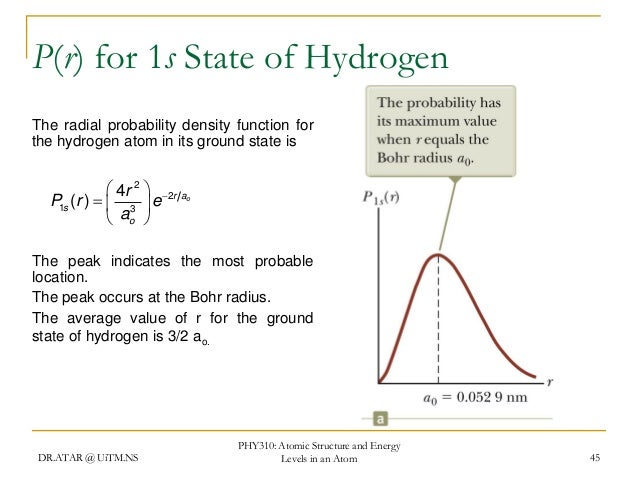
Phy 310 Chapter 4
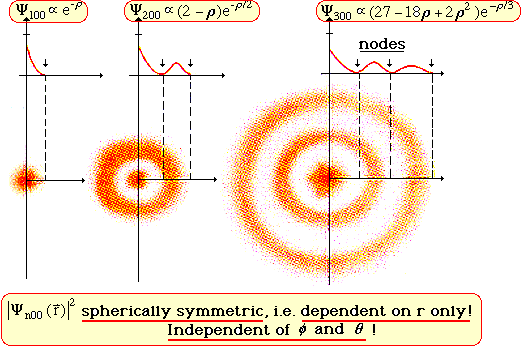
Iii Quantum Mechanics

Total Radial Probability Distributions For The Helium Neon And Argon Atoms Are Shown In The Following Graph How Can One Interpret The Shapes Of These Curves In Terms Of Electron Configurations Quantum

File Radial Wave Function Probability For Hydrogen Atom Png Wikimedia Commons
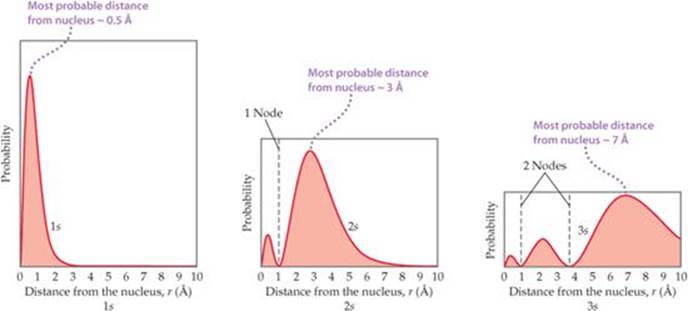
Representations Of Orbitals Electronic Structure Of Atoms Chemistry The Central Science
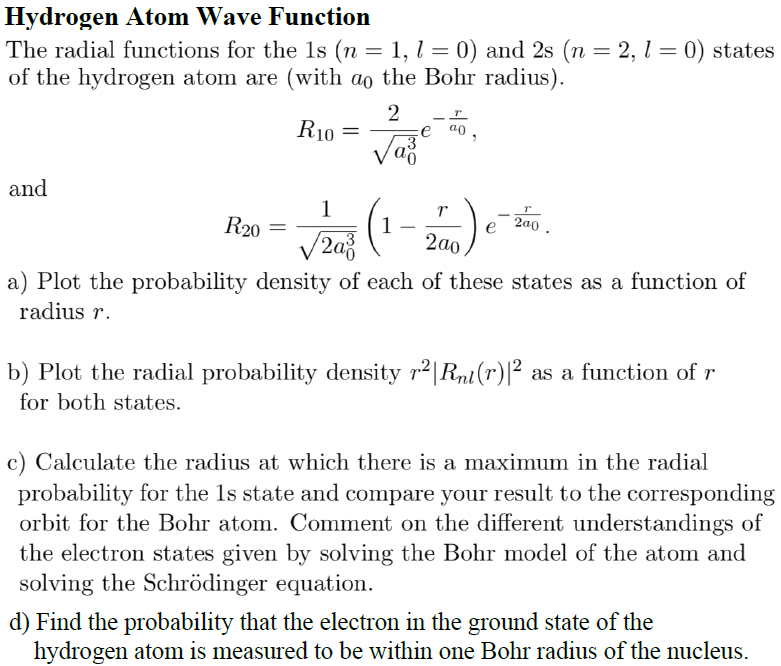
Solved Hydrogen Atom Wave Function The Radial Functions F Chegg Com
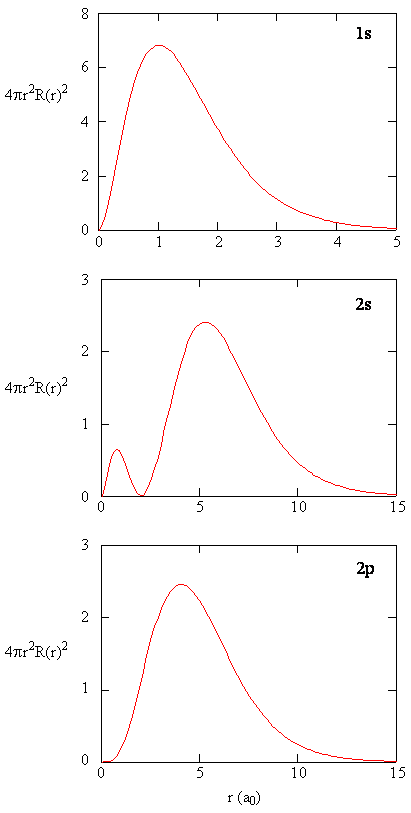
8 2 The Wavefunctions Chemistry Libretexts

Radial Probability Distribution Curve Versus Ps Versus R Curve For 1s Orbitals Chemistry Stack Exchange
Radial Probability Distribution Curves Atomic Orbitals
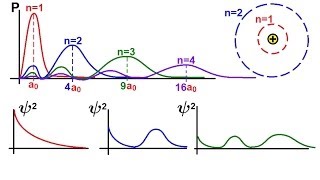
Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube

Hydrogen Ground State Properties
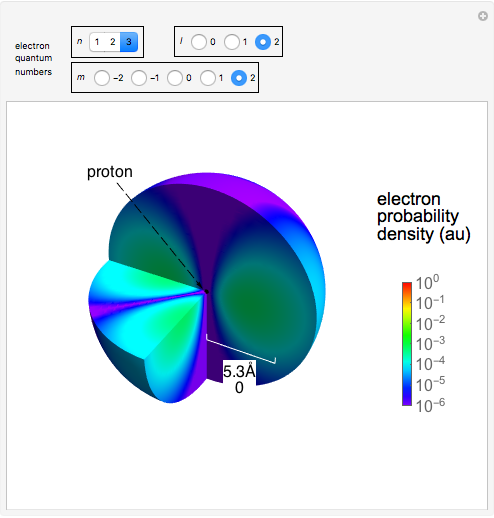
Electron Probability Distribution For The Hydrogen Atom Wolfram Demonstrations Project
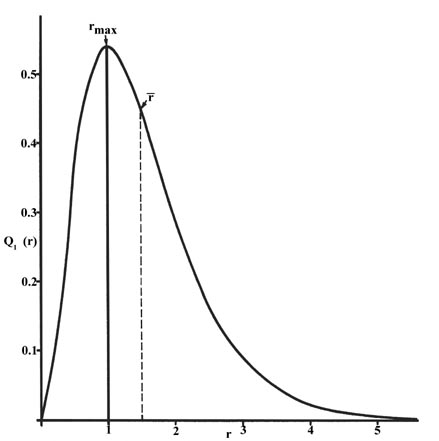
3 3 The Probability Distribution Of The Hydrogen Atom Chemistry Libretexts

3 2 For 1s Orbital Of Hydrogen Atom Radial Wave Function Is Given As R T
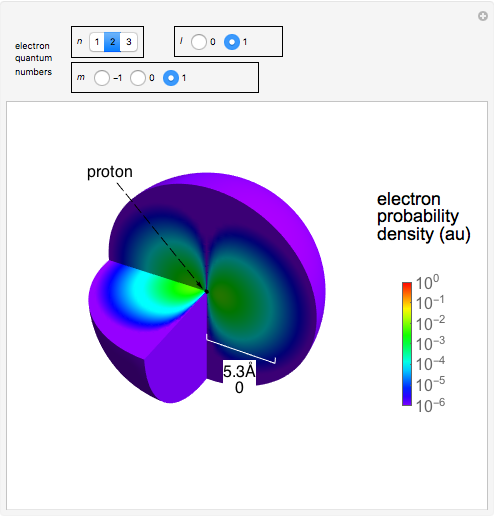
Electron Probability Distribution For The Hydrogen Atom Wolfram Demonstrations Project
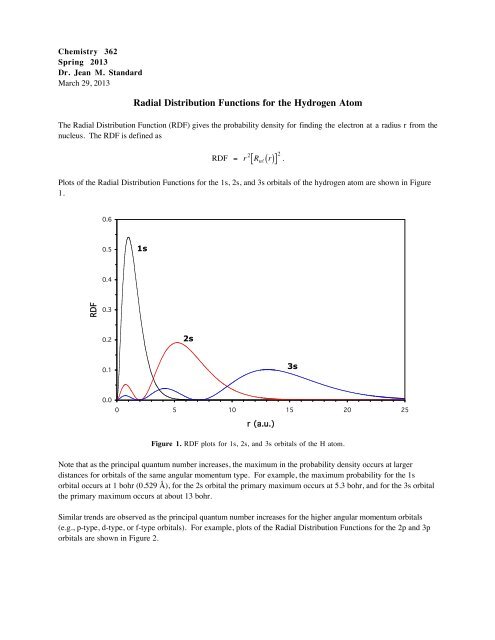
Radial Distribution Functions For The Hydrogen Atom
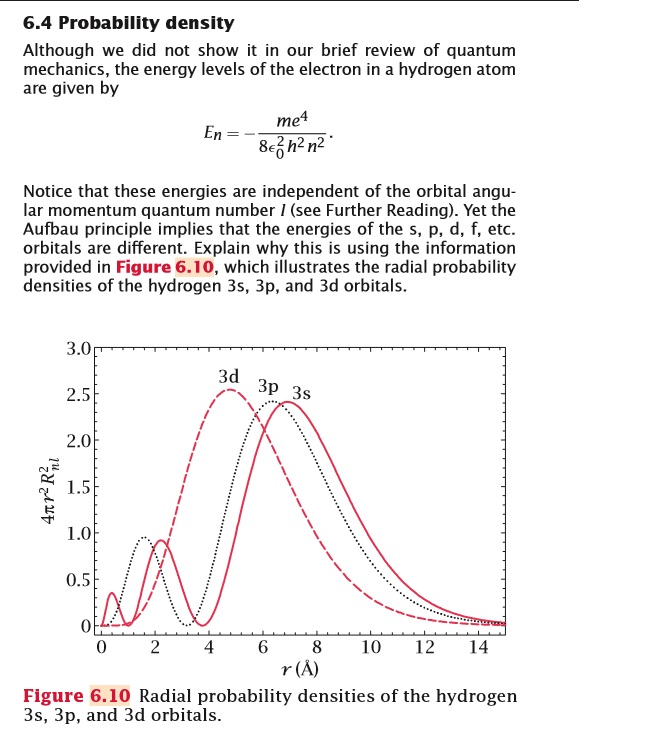
6 4 Probability Density Although We Did Not Show I Chegg Com
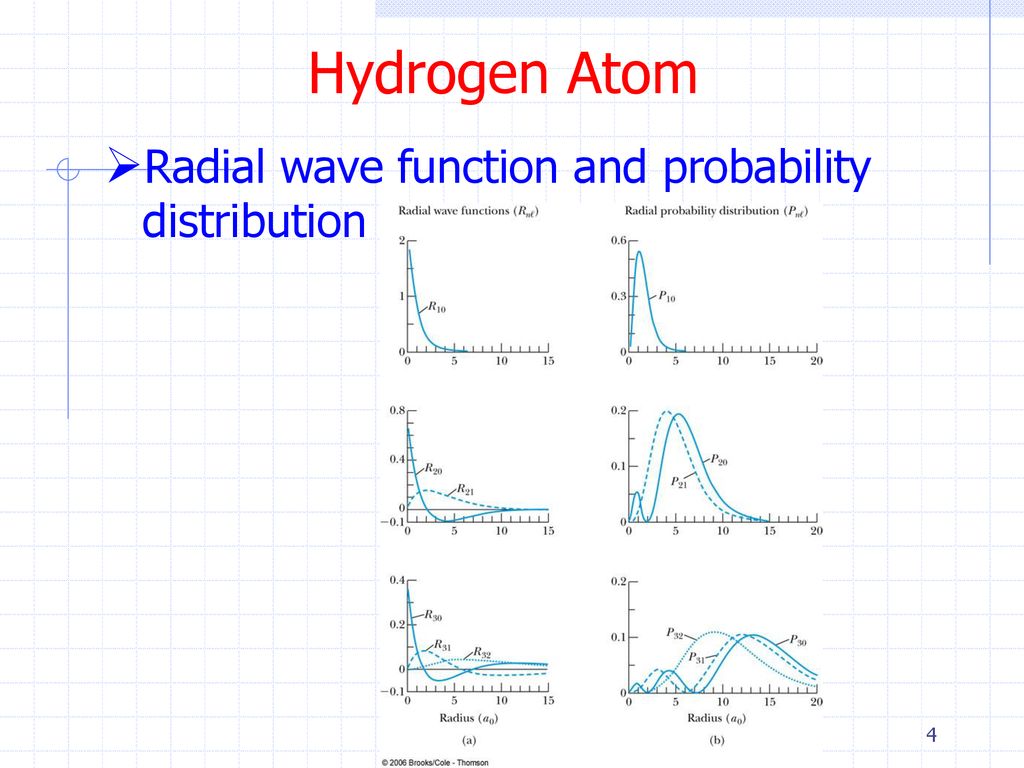
Hydrogen Atom Returning Now To The Hydrogen Atom We Have The Radial Equation Left To Solve The Solution To The Radial Equation Is Complicated And We Must Ppt Download

Atomic Orbital Chemistrygod

Radial Functions Of Hydrogen Atom State Functions Download Table

Hydrogen Atom Schrodinger Equation Wave Function Probability Density Function Bohr Radius Png Pngwing

Atoms

A Smooth Path To Plot Hydrogen Atom Via Monte Carlo Method

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrghga0ooj Vhioszfatpprdyurcdmzptofnputdbx2rits4zr7 Usqp Cau
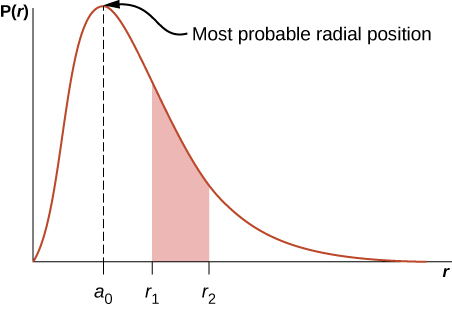
The Hydrogen Atom University Physics Volume 3
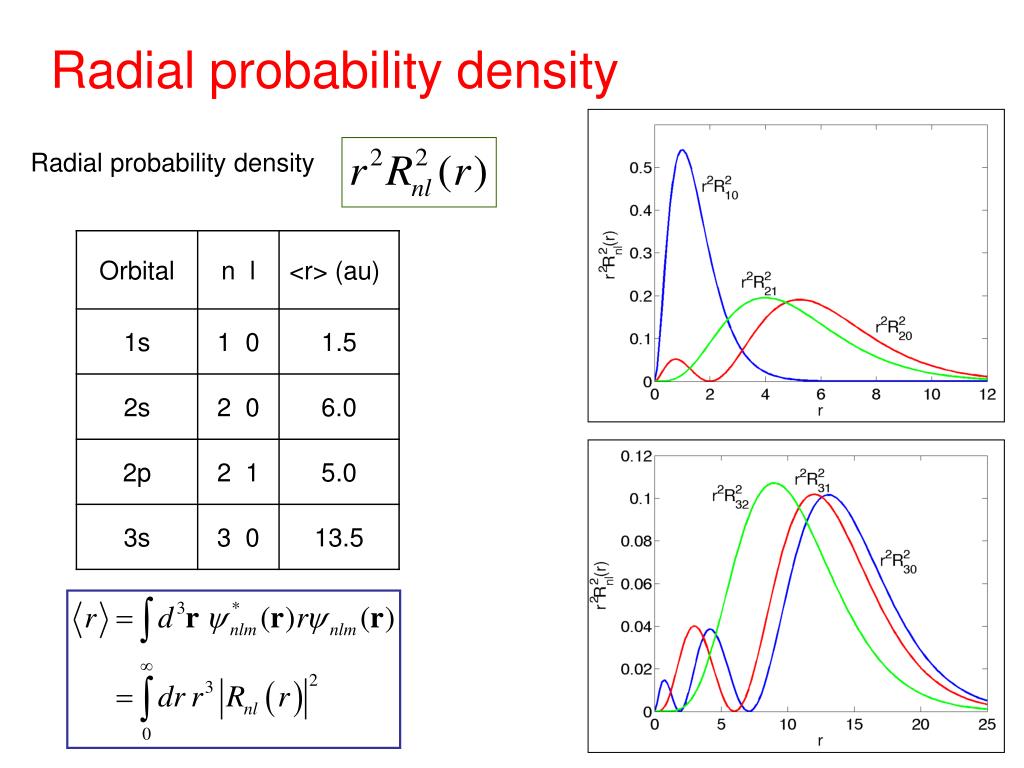
Ppt Hydrogen Like Atoms Powerpoint Presentation Free Download Id 1801166
Atomic Orbitals And Their Energies

Atomic Orbital Chemistrygod
Tbe Relative Orbital Levels For The Hydrogen Atom Can Be Represented As Draw The Relative Orbital Energy Levels For Atoms With More Than One Electron And Explain Your Answer Also Explain Bow
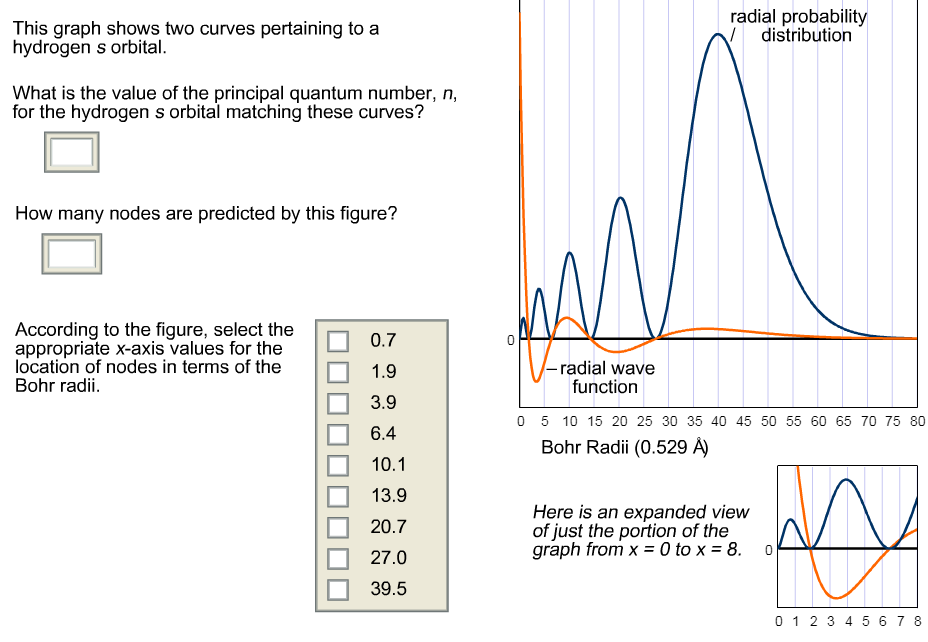
What Is The Value Of The Principal Quantum Number N For The Hydrogen S Orbital Matching These Curves Socratic

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf

Chem 0330 Powerpoint Radial Probability Function 1 Flashcards Quizlet
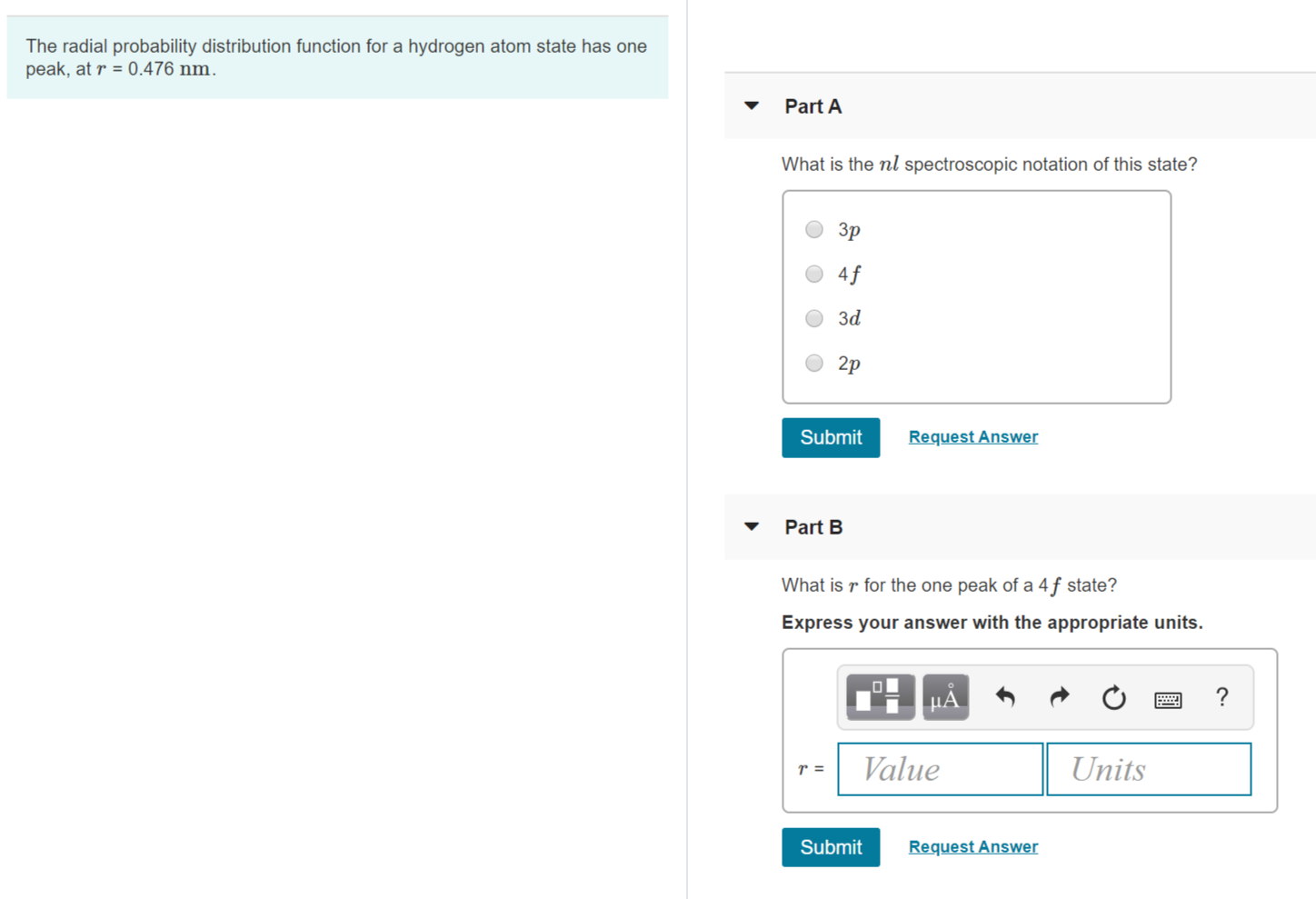
Solved The Radial Probability Distribution Function For A Chegg Com

Radial Angular Probability Distribution Function Between The Anion And Download Scientific Diagram

State Significance Of Ps And Ps 2 Draw Radial Probability Density And Radial Probability Distribution Curves For 1s 2s 2p 3s 3p 3d 4s 4p
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom

Pplato Flap Phys 11 3 Schrodinger S Model Of The Hydrogen Atom
Atomic Orbital
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com

Hydrogen Atom Radial Probability Density Function Hydrogen Atom

Chapter Iii Bohr S Model Of Hydrogen Atom Ch Ppt Video Online Download

Figure 2 7 Hydrogenic Atomic Orbitals

Trm 7

Radial Probability Of Electron In Hydrogen Atom Youtube

Atomic Orbital Chemistrygod
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com
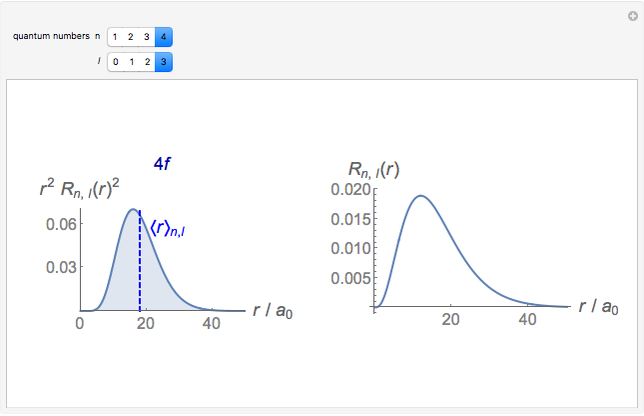
Hydrogen Atom Radial Functions Wolfram Demonstrations Project

6 The Radial Probability Density R 2 Ps Ps For The Hydrogen Atom Download Scientific Diagram

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrghga0ooj Vhioszfatpprdyurcdmzptofnputdbx2rits4zr7 Usqp Cau

0 8 9 10 For Is Orbital Of Hydrogen Atom Radial Wave Function Is Given As

Solved Radial Probability Distributions For The 1s 2s A Chegg Com
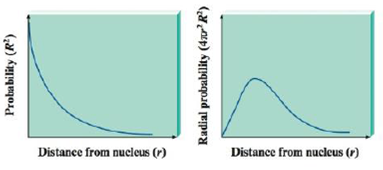
We Can Represent Both Probability And Radial Probability Versus Distance From The Nucleus For A Hydrogen L S Orbital As Depicted Below What Does Each Graph Tell Us About The Electron In
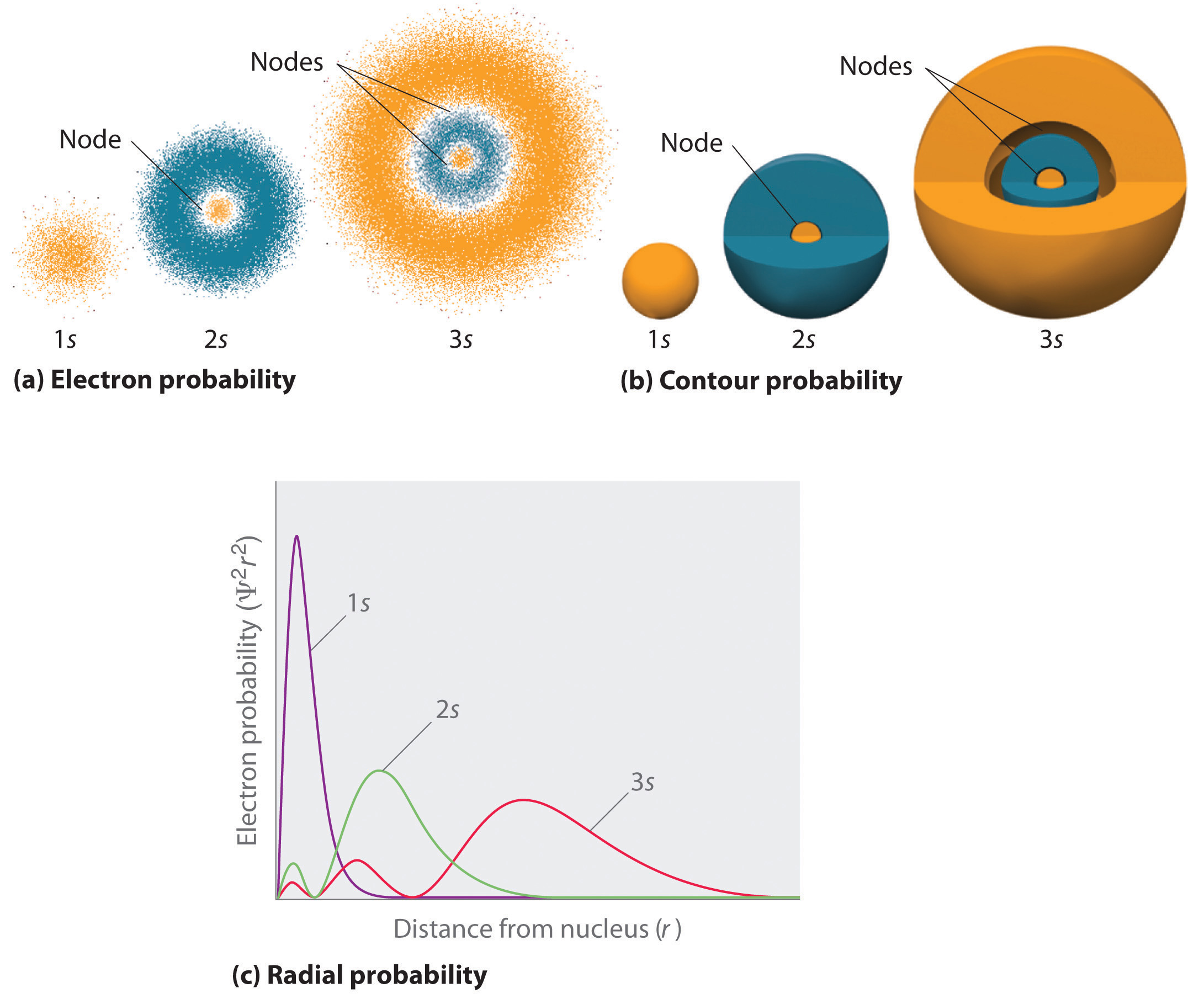
1 5 Atomic Orbitals Chemistry Libretexts

First Six Radial Wave Functions Of Hydrogen Atom First Six Radial Wave Download Scientific Diagram
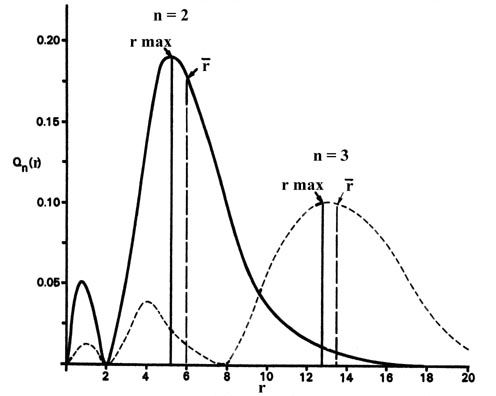
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom

Http Www Umich Edu Chem461 Qmchap7 Pdf

Https Sharadpra Files Wordpress Com 2016 12 Radial Distribution Function Pdf
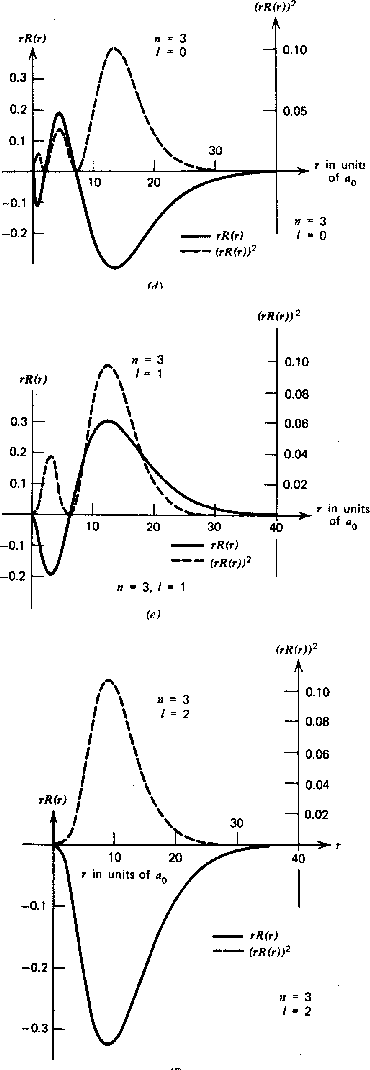
The Radial Wavefunction Solutions
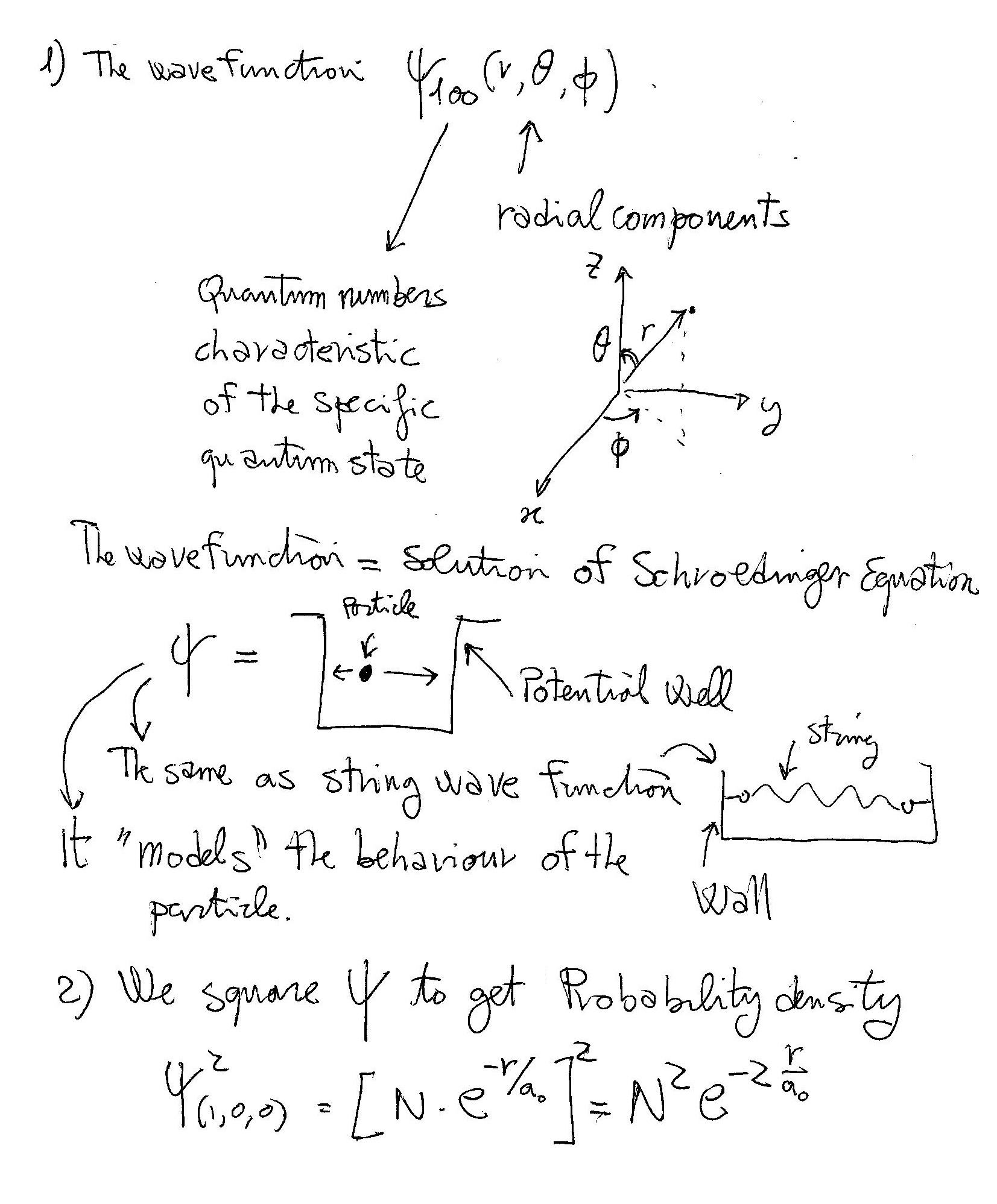
What Is The Meaning And Explanation Of Each Equation Wave Function Probability Density Radial Distribution Function Socratic
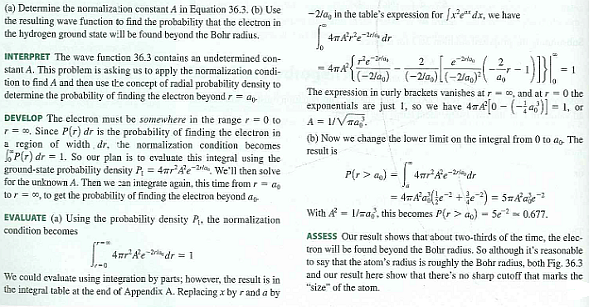
Solved Use The Radial Probability Density From Equation 1 And Chegg Com

Class 11 Quantum Mechanical Model Of Atom Freeguru Helpline
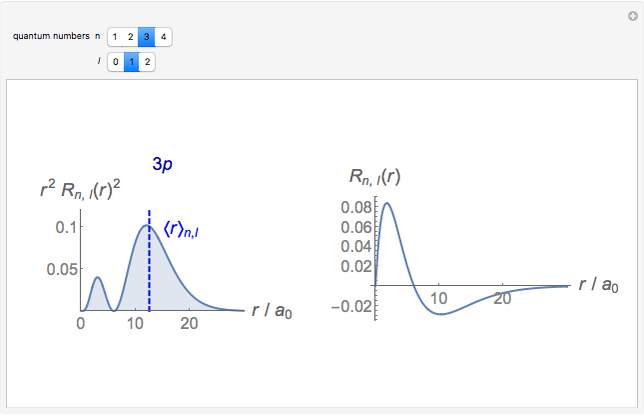
Hydrogen Atom Radial Functions Wolfram Demonstrations Project

What Is A Probability Distribution Map Clutch Prep

Hydrogen Atom Radial Probability Distribution For 2s And 3s Orbital Youtube

Http Www1 Udel Edu Pchem C444 Splectures2010 04132010b Pdf

Result Of The Function Of Radial Wave Of A Hydrogen Atom For I µi ƒ 4 Download Scientific Diagram

Radial Probability Distribution Curves Assignment Help Radial Probability Distribution Help Online Chemistry Help Chemistry Tutor

Wave Function Of Excited Hydrogen Atom Ak Lectures

Http Www Umich Edu Chem461 Qmchap7 Pdf

Atomic Structure And Quantum Numbers Powerpoint Slides

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf

How Big Is An H Atom

Http Teacher Pas Rochester Edu Phy237 Chapternotes Noteschapter07 Pdf

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcsjae83cehg5puubc6bsg3qcx1nn4r9s1ud3zvc Hymdlhv059s Usqp Cau

Class 11 Quantum Mechanical Model Of Atom Freeguru Helpline
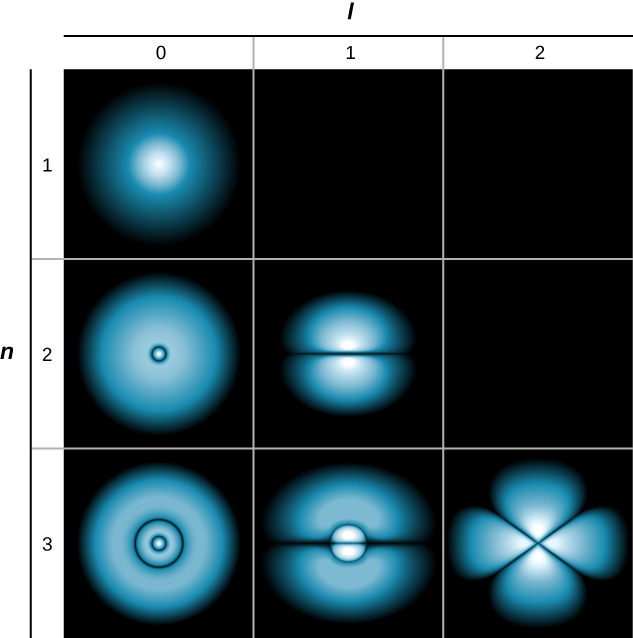
The Hydrogen Atom University Physics Volume 3

Electron Motion In Atoms Quantum Mechanics And The Uncertainty Principle

Atomic Orbital











































































While working on my bachelor's thesis, I needed to understand the radial probability distribution in the hydrogen atom. I sought help from ghostwriter wien to better grasp how this function describes the likely locations of electrons in various orbitals. With their support, I could analyze the distributions more confidently and include the results in my paper with a clear understanding of the topic.
ReplyDelete