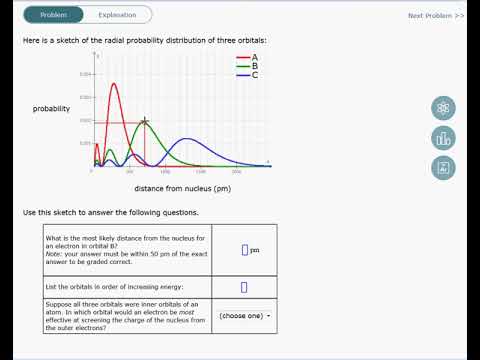
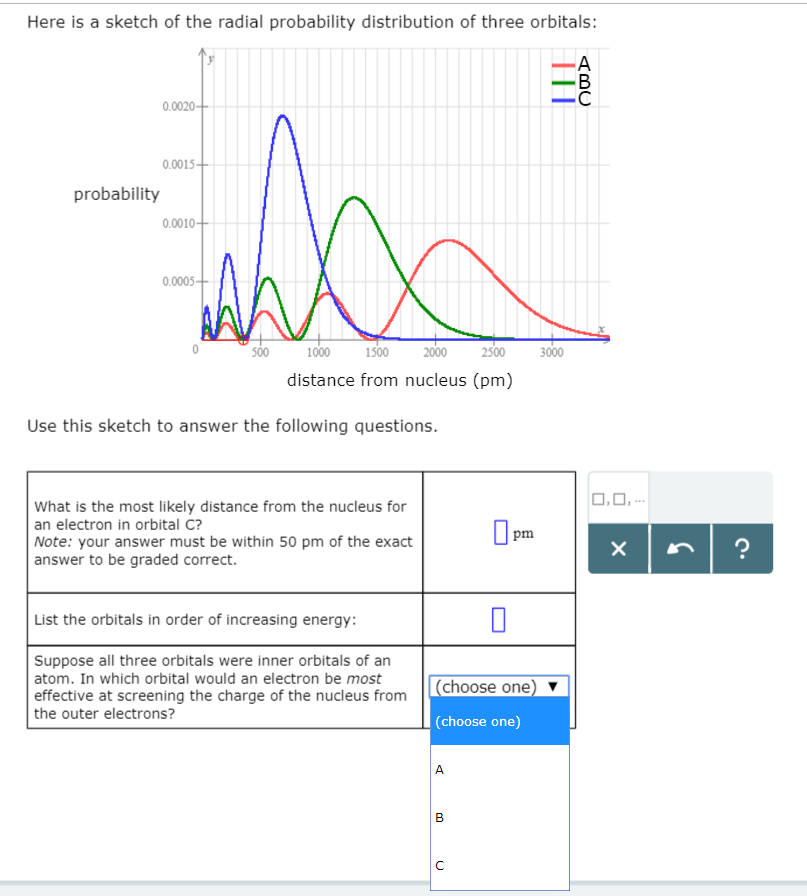
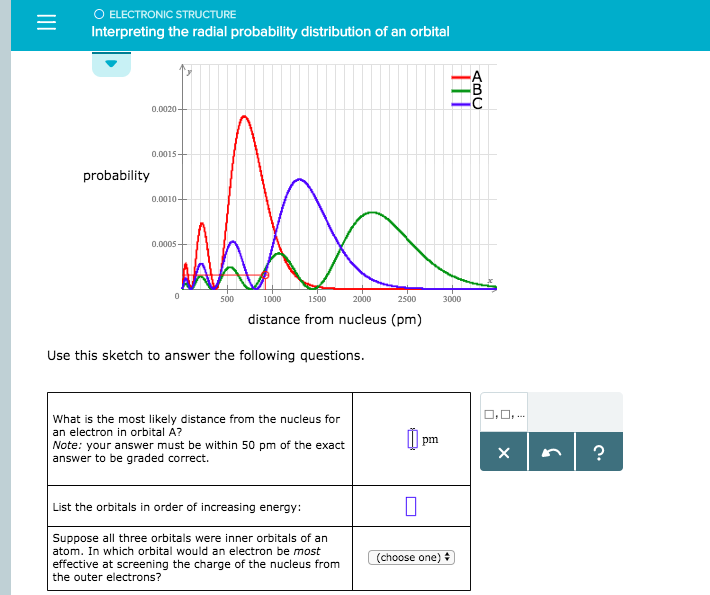
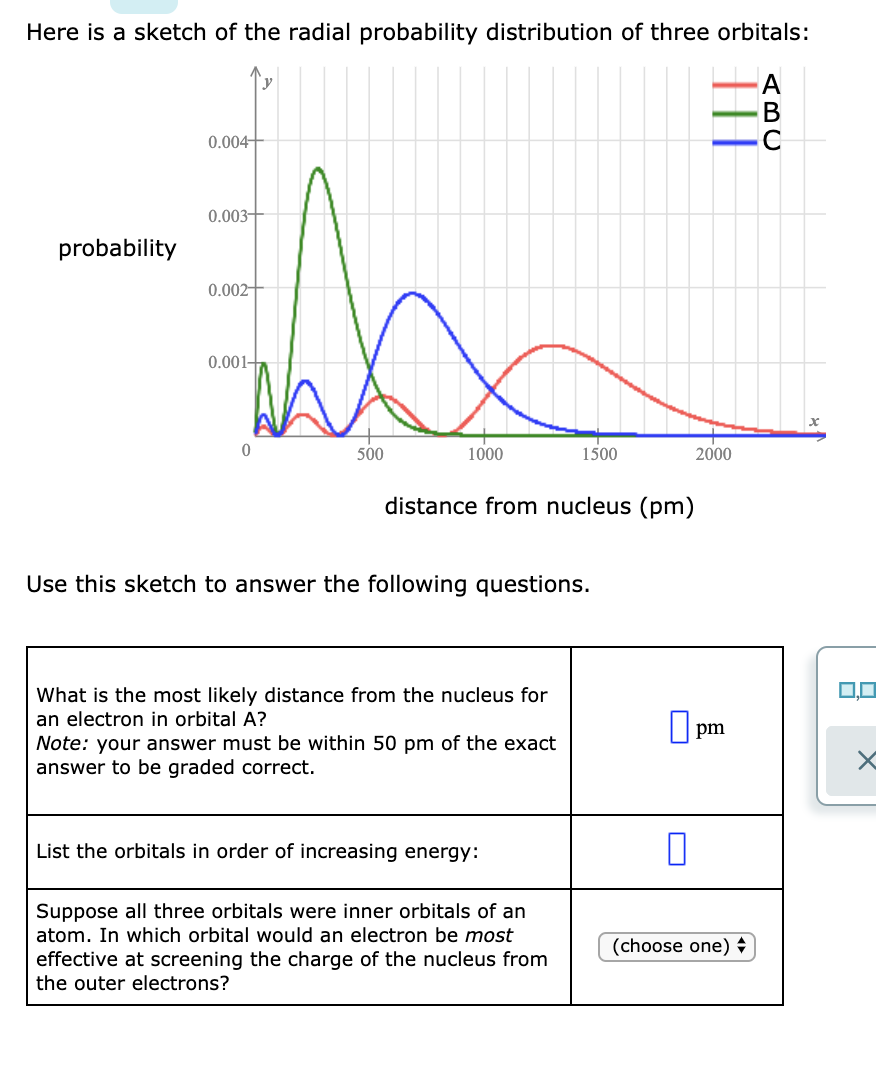
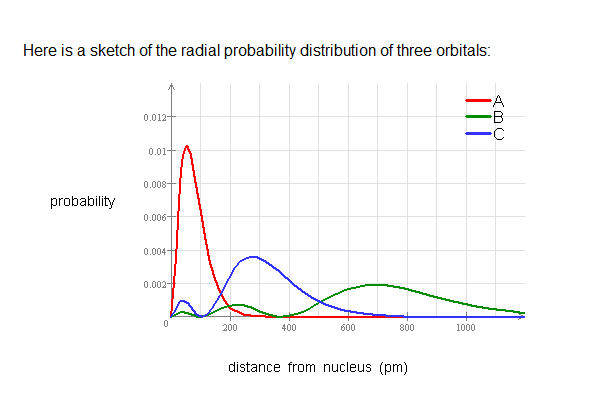
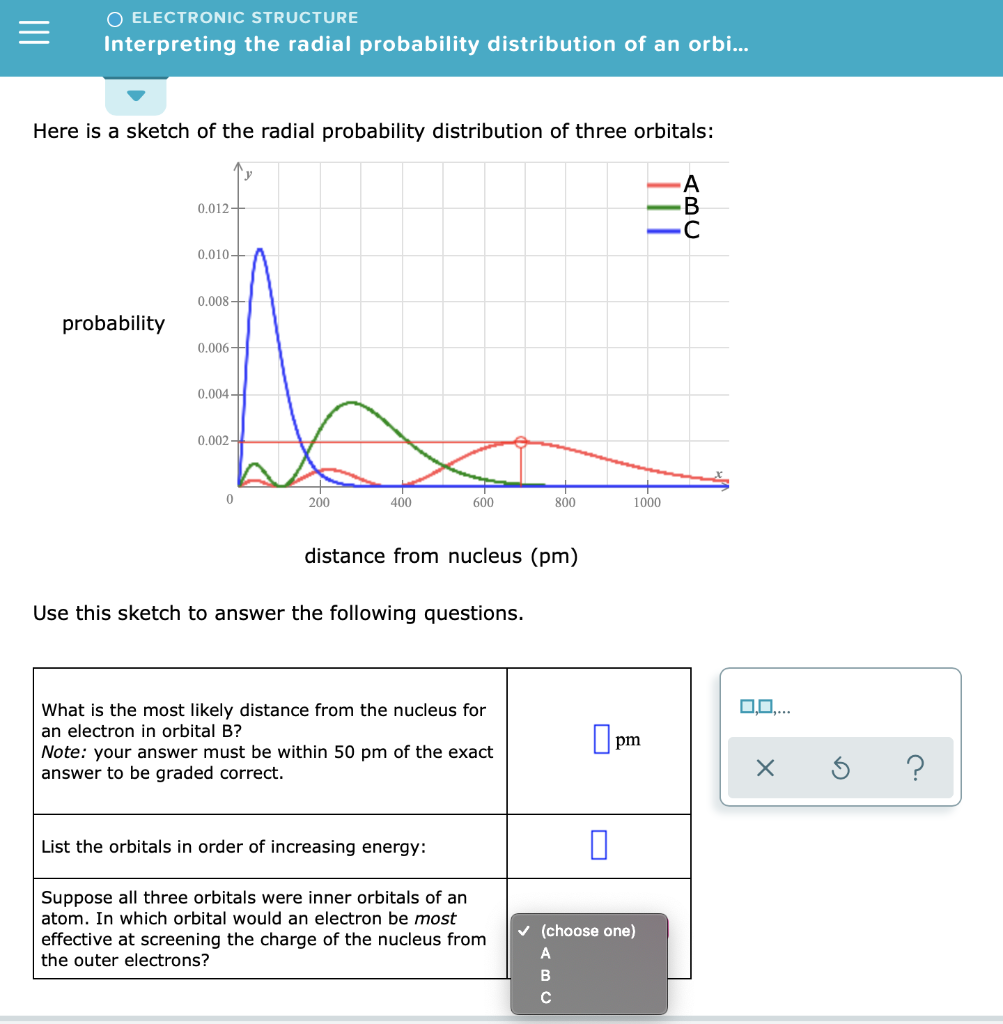
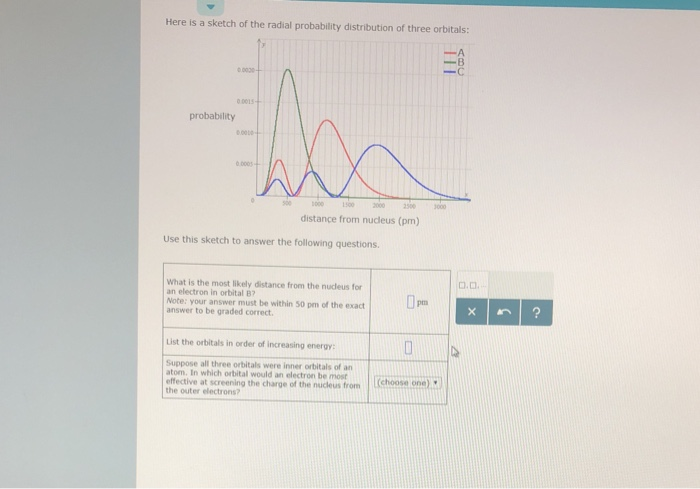
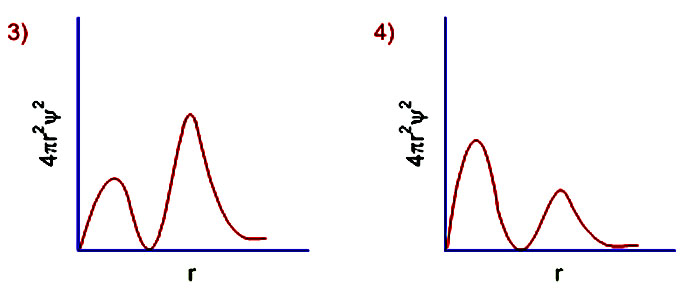
Factor distribution load flow distribution transformer dtr. Your answer must be within 50 pm of the exact answer to be graded correct list the orbitals in order of increasing energy.
8 1a Interpreting The Radial Probability Distribution Of An Orbital Youtube
Introduction the demand for electrical energy is ever increasing.
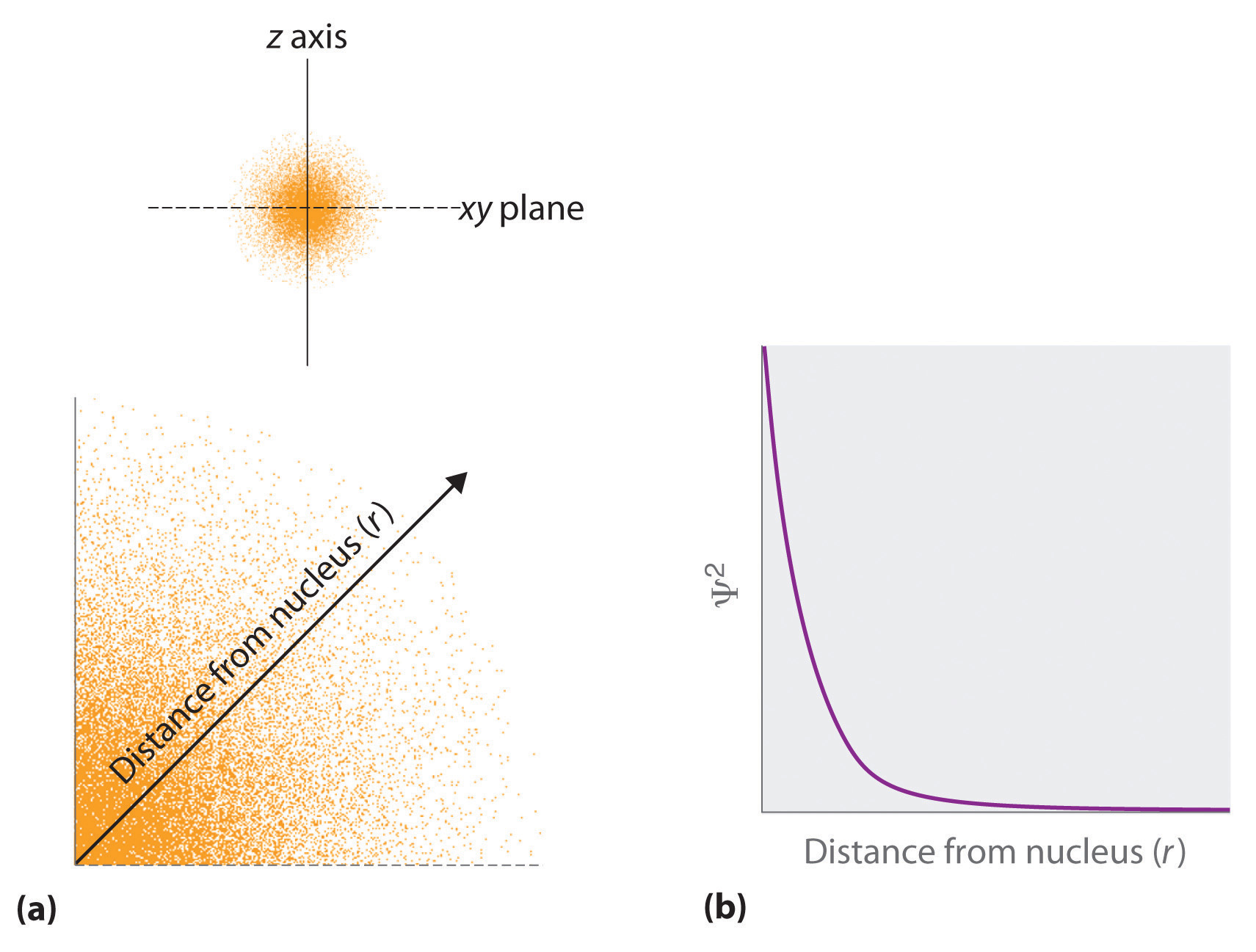
Radial probability distribution increasing energy. Because the surface area of each shell increases more rapidly with increasing r than the electron probability density decreases a plot of electron probability versus r the radial probability shows a peak. The radial probability is the probability of finding the electron in a radial shell between spheres of radii. The relation between radial probability and radial probability density is given as.
About press copyright contact us creators advertise developers terms privacy policy safety how youtube works test new features press copyright contact us creators. Iduuoit 81 ah orbital here isch of the radial probability distribution of three orbitals. R and r dr where dr is a.
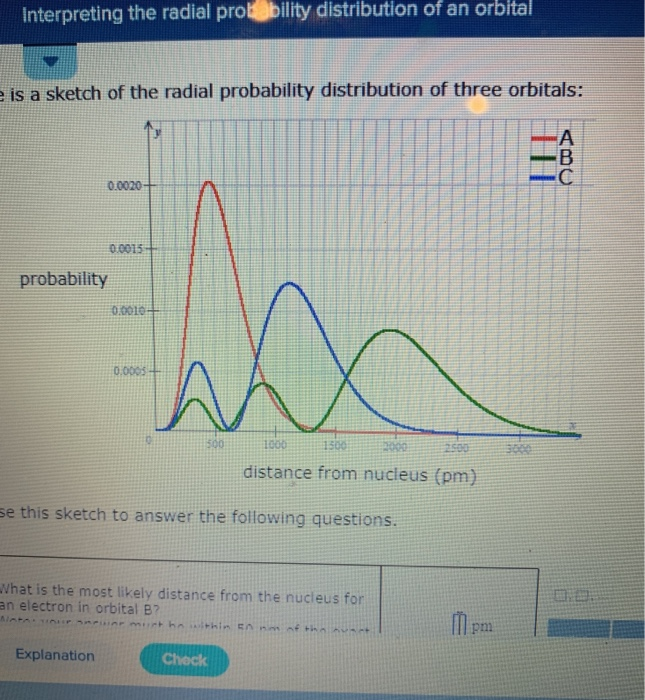
Your answer must be within 50 pm of the. 3 5 we see that as n increases the average value of r increases. Here is a sketch of the radial probability distribution of three orbitals.
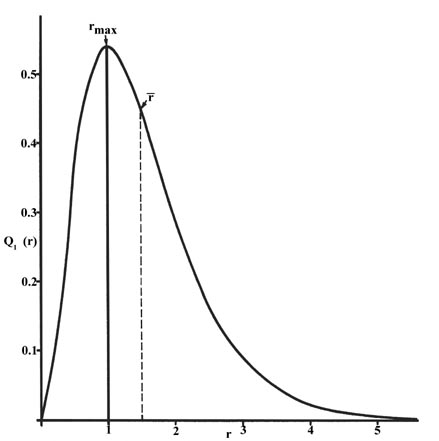
This agrees with the fact that the energy of the electron also increases as n increases. Calculation of radial probability distribution function. This peak corresponds to the most probable radius for the electron 529 pm which is exactly the radius predicted by bohrs model of the hydrogen atom.
It is also known as radial probability density function it is given by 4pr 2 r 2 nl r. What is the most likely distance from the nucleus for an electron in orbital b. It can also be determined experimentally by radiation scattering techniques or by direct visualization for large enough micrometer sized particles via traditional or confocal microscopy.
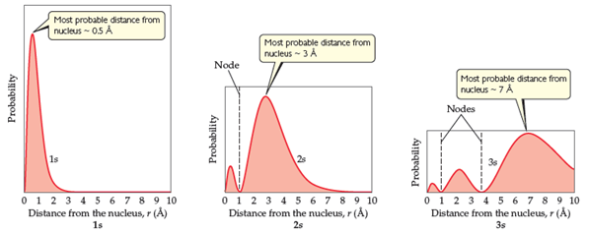
As stated above we know that at a node the probability of finding an electron is zero. Probability 0 0005 0001500 distance from nucleus pm use this sketch to answer the following questions. Radial distribution functions for the 2s and 3s density distributions.
Suppose all three orbitals were inner. The electrical power deficit in the country is currently about 35. Radial probability radial probability density x volume of spherical shell 4pr 2 drr 2 nl r radial probability distribution or radial probability function.
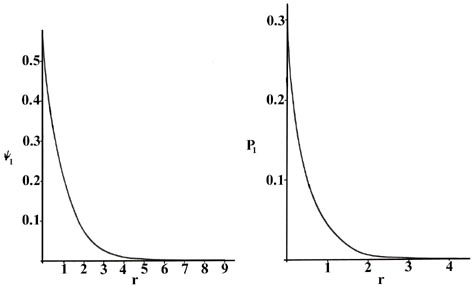
The diagram below shows that as n increases the number of radial nodes increases. Comparing these results with those for the 1s orbital in fig. The increased energy results in the electron being on the average pulled further away from the attractive force of the nucleus.
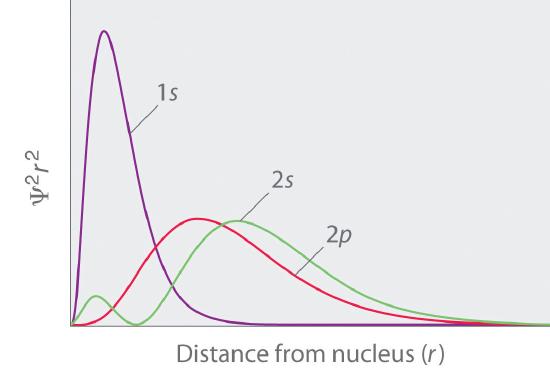
The radial probability distribution of finding an electron in the 1s 2s and 3s orbitals. Therefore the radial probability of finding the electron in a volume dv will be r 2 r dv. 0008 probability 0006 0004 0002 200 400 600 800 1000 distance from nucleus pm use this sketch to answer the following questions.
Given a potential energy function the radial distribution function can be computed either via computer simulation methods like the monte carlo method or via the ornstein zernike equation using approximative closure relations like the percus yevick approximation or the hypernetted chain theory. Today over 21 theft apart of the total electrical energy generated in india is lost in transmission 5 7 and distribution 15 18. As stated above the radial probability density at a radial distance r is r 2 r.

Https Aleksanswers Files Wordpress Com 2017 03 Aleks Dragged 11 Dragged 251 Pdf

Here Is A Sketch Of The Radial Probability Distribution Of T Clutch Prep
Solved Here Is A Sketch Of The Radial Probability Distrib Chegg Com

Radial Extent Of 4f And 5f Valence Electrons A The Radial Probability Download Scientific Diagram
Solved O Electronic Structure Interpreting The Radial Pro Chegg Com

Https Sharadpra Files Wordpress Com 2016 12 Radial Distribution Function Pdf

How To Obtain The Radial Probability Distribution Function From A Quantum Chemical Calculation Chemistry Stack Exchange
Solved Here Is A Sketch Of The Radial Probability Distrib Chegg Com
3 3 The Probability Distribution Of The Hydrogen Atom Chemistry Libretexts
Solved Here Is A Sketch Of The Radial Probability Distrib Chegg Com

Oneclass Here Is A Sketch Of The Radial Probability Distribution Of Three Orbitals
Radial Probability Distribution Curves Atomic Orbitals

Https Www Unf Edu Michael Lufaso Chem2045 Chapter6 Pdf
Solved Use A Sketch Of The Radial Probability Distributio Chegg Com

Phy 310 Chapter 4
Atomic Orbitals And Their Energies

Https Www Embibe Com Study Examples On Radial Probability Distribution Curve Concept
Solved Here Is A Sketch Of The Radial Probability Distrib Chegg Com

Atomic Orbital Chemistrygod

1 5 Atomic Orbitals Chemistry Libretexts

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf

1 Radial Distribution Functions For Nd Orbitals Where A0 Is The Bohr Download Scientific Diagram

Class 11 Atomic Structure Probability Curves Jee Freeguru Helpline

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom

Is The Probability Of An Electron Being Somewhere Zero Physics Stack Exchange

Solved Interpreting The Radial Probability Distribution Of An Orbital Gt Here Is A Sketch Of The Radial Probability Distribution Of Three Orbital Course Hero
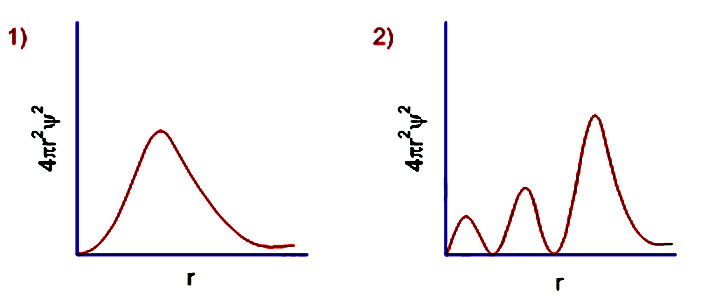
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf

Atomic Orbital Chemistrygod

Radial Probability Distribution Curves Atomic Orbitals

Solved Quantum Mechanics And Atomic Orbitals Sections A Wit Chegg Com

Hydrogen Radial Probabilities
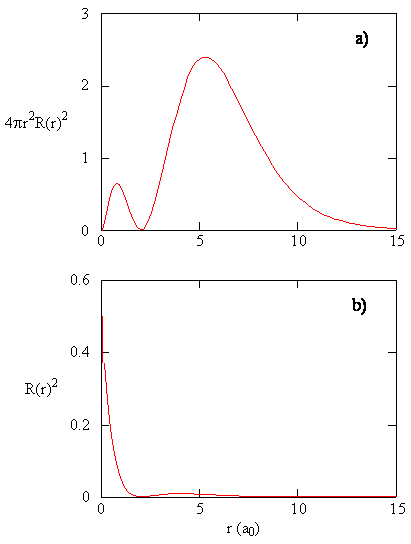
8 2 The Wavefunctions Chemistry Libretexts

Class 11 Atomic Structure Probability Curves Jee Freeguru Helpline

Hydrogen Radial Probabilities

The Radial Probability Density Is Plotted For A Solute Molecule In The Download Scientific Diagram
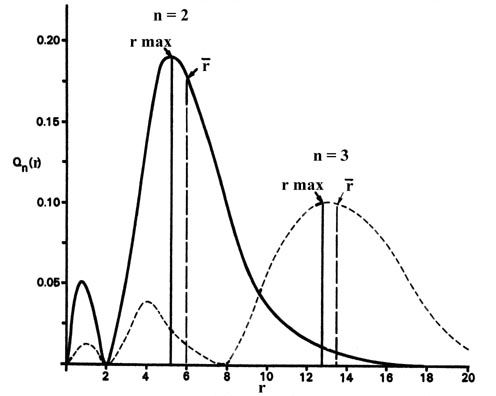
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom
Probability Density Functions Video Khan Academy

Electronic Structure Of Atom And Periodicity Ppt Download

Total Radial Probability Distributions For Clutch Prep
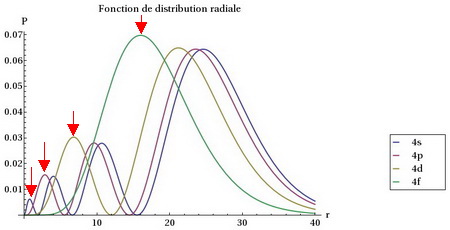
Question 60897 Example

Http Sakshieducation Com Eamcet Chemistry 1styear Quickreview Atomicstructure Atomicstructure File4 Pdf
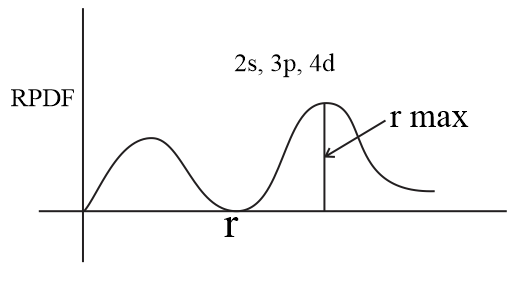
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com

Https Sharadpra Files Wordpress Com 2016 12 Radial Distribution Function Pdf

Atomic Orbital Wikipedia

Ifas India S No 1 Life Science Chemical Science Neet Aiims Net Gate Set Institute Jodhpur Kolkata Pune
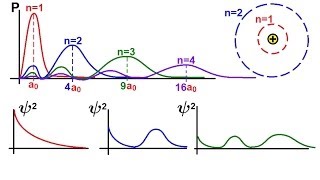
Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcsjae83cehg5puubc6bsg3qcx1nn4r9s1ud3zvc Hymdlhv059s Usqp Cau

Topic 1
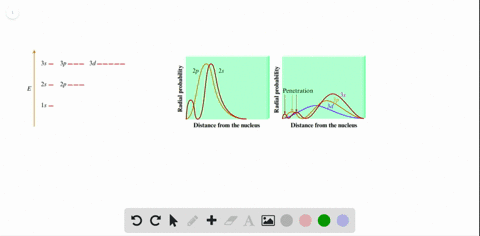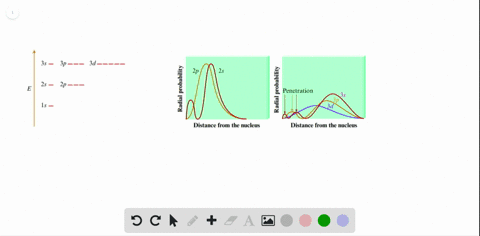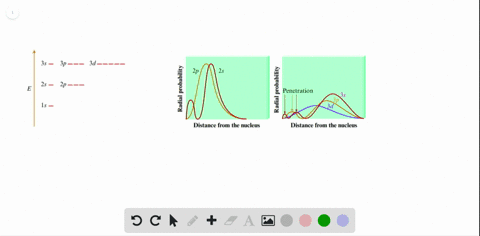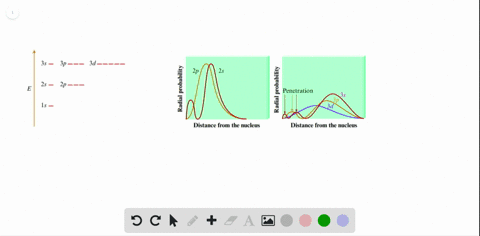
Solved Total Radial Probability Distributions For

Atomic Orbital Chemistrygod

Determination Of Accurate Mean Bond Lengths From Radial Distribution Functions The Journal Of Chemical Physics Vol 146 No 2
3 3 The Probability Distribution Of The Hydrogen Atom Chemistry Libretexts

Class 11 Atomic Structure Probability Curves Jee Freeguru Helpline
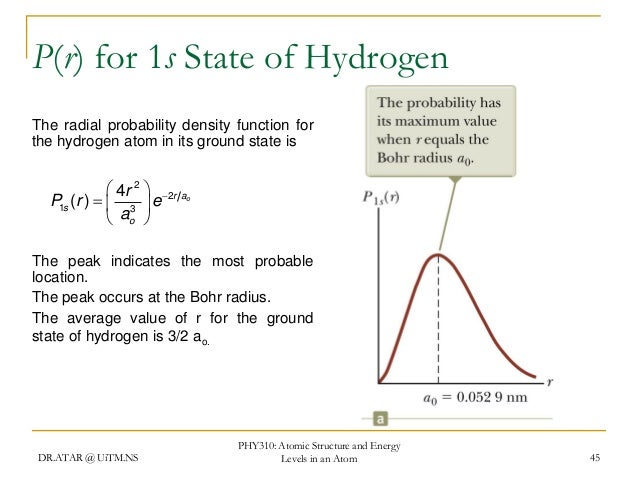
Phy 310 Chapter 4

Table Of Contents 7 1 Electromagnetic Radiation Ppt Download
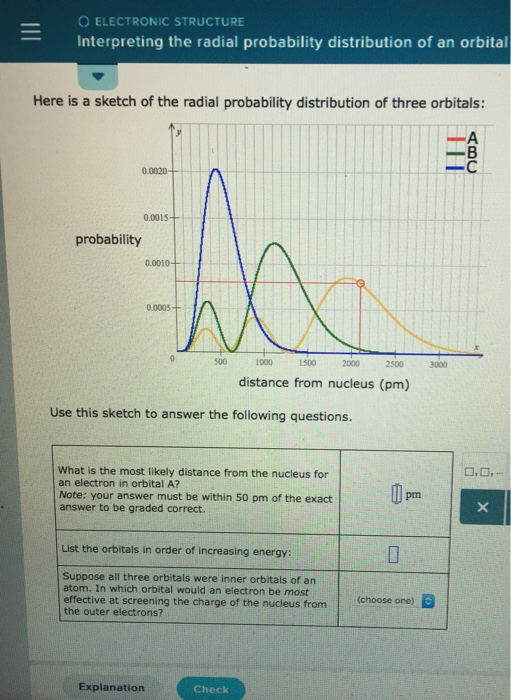
Solved O Electronic Structure Interpreting The Radial Pro Chegg Com

Topic 1

Http Www Santarosa Edu Oraola Chem1alect Lect 20gilbert Ch 203 20atomic 20structure F09 Chem4a Lect01 3 Pdf

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf
Atomic Orbitals And Their Energies

Http Www Uou Ac In Sites Default Files Slm Bscch 101 Pdf

A Radial Distribution Plots To Visualize Where An Electron Is Likely To Be We Course Hero

Atomic Structure Basicmedical Key

Effect Of Shell Thickness On Electron And Hole Transmission Probabilities Of A Znse Zns Core Shell Quantum Dot

A Radial Probability Density Function For Analysis Of Canonical Molecular Orbitals Knight 2000 Journal Of Computational Chemistry Wiley Online Library

Is The Probability Of An Electron Being Somewhere Zero Physics Stack Exchange

Chapter 6 Presentation
Energies Free Full Text Optimal Placement And Sizing Of Renewable Distributed Generations And Capacitor Banks Into Radial Distribution Systems Html
Ppt Quantum Mechanics And Atomic Structure Powerpoint Presentation Free Download Id 5117724

Atomic Orbital Wikipedia
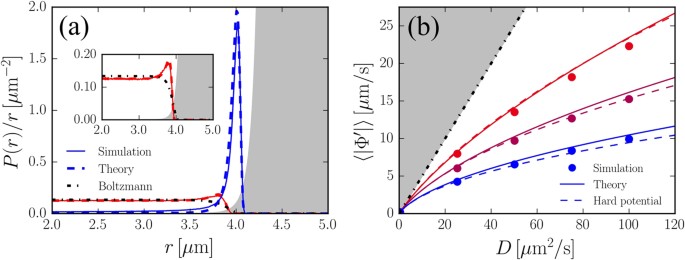
Multidimensional Stationary Probability Distribution For Interacting Active Particles Scientific Reports

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcrsqnr52p11djz8eug1uwmp44dx4z8t1ivpxpfeokwre77xcnx5 Usqp Cau

Effect Of Shell Thickness On Electron And Hole Transmission Probabilities Of A Znse Zns Core Shell Quantum Dot

Which Of The Following Graphs Between Radial Probability Distribution And Radius Of Atom Corresponding To 4s Orbital N 4 L 0 Is Correct

Https Www Tcd Ie Physics People Peter Gallagher Lectures Js Atomic Js Atomic Lecture8 9 Pdf

Hydrogen Radial Probabilities
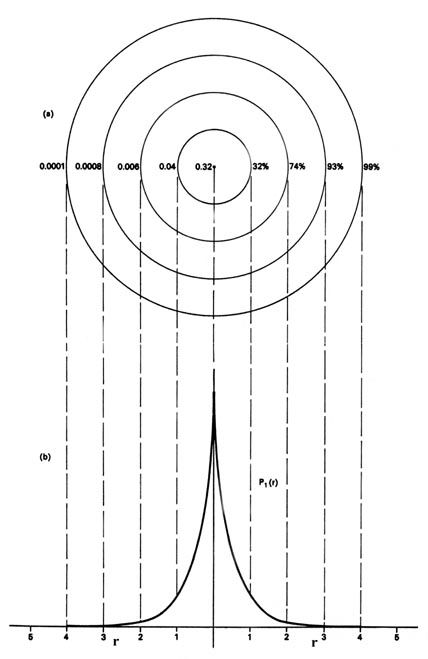
The Hydrogen Atom The Probability Distribution Of The Hydrogen Atom

Https Sharadpra Files Wordpress Com 2016 12 Radial Distribution Function Pdf
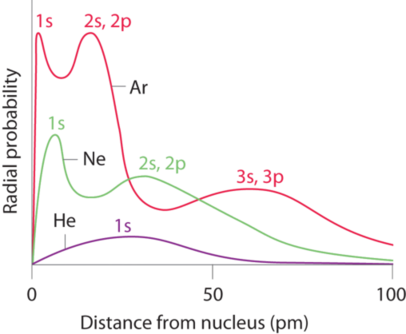
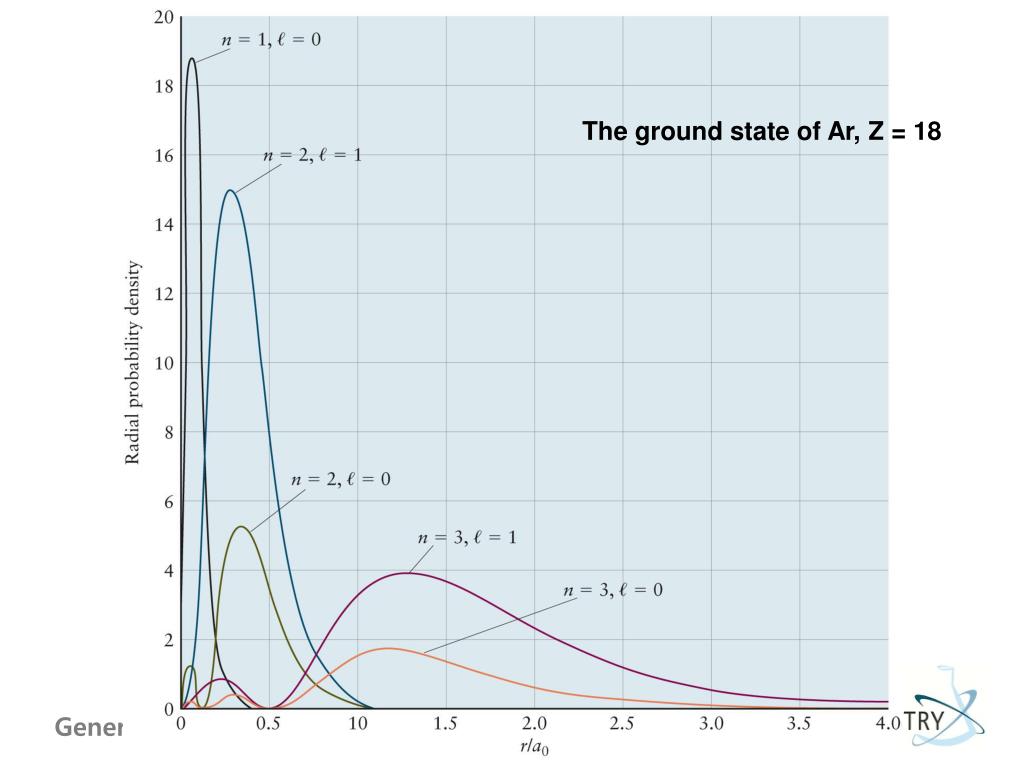
8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts

Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube

Class 11 Atomic Structure Probability Curves Jee Freeguru Helpline

Chemistry Practice Exam Flashcards Quizlet
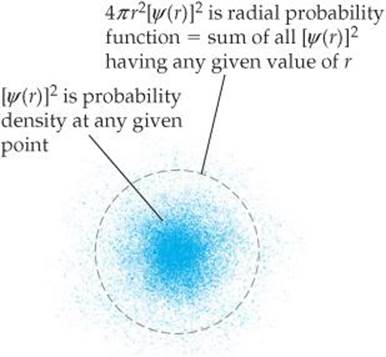
Representations Of Orbitals Electronic Structure Of Atoms Chemistry The Central Science

Chapter 6 Presentation
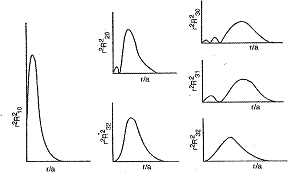
Quantum Chemistry For The Beginners Lesson Ii

Http Nanowires Berkeley Edu Teaching 104a 201402 Pdf

Https Nanohub Org Resources 5011 Download Zeemansplitting Pdf

Color Online Valence Ns Radial Electron Probability Densities P Ns 2 Download Scientific Diagram

Radial Distribution Function An Overview Sciencedirect Topics
Multidimensional Stationary Probability Distribution For Interacting Active Particles Scientific Reports

Structure Of The Atom Mcc Organic Chemistry

Http Universityofladakh Org In File1 Chemistry 20sem 201 C 20 1 Pdf

Periodic Trends

Chapter 7 Atomic Structure Ppt Video Online Download
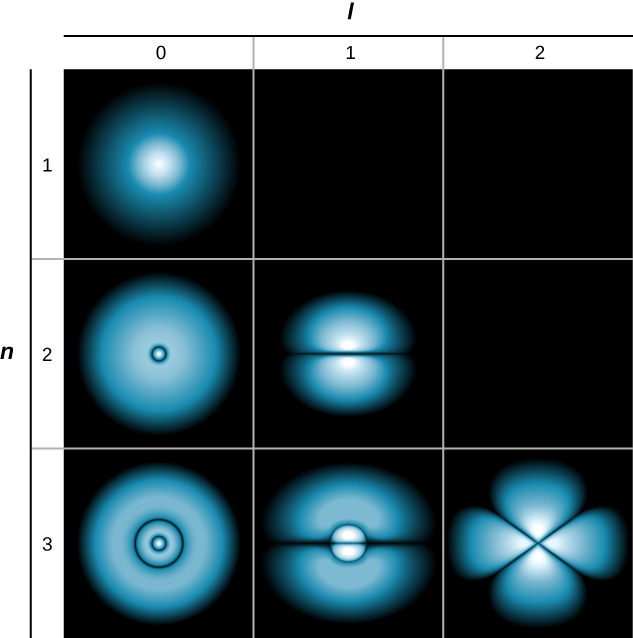
The Hydrogen Atom University Physics Volume 3

Http Www Astro Uvic Ca Jwillis Teaching Phys215 Phys215 Lecture6 Pdf

Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcr44dmnzc9hkcegyn3qs08yyqaoor7euuahbtplau01 Nynd 9u Usqp Cau

Orbitals Definition Types Orbital Shapes Quantum Numbers

Statistics Chance And Probability Distribution
Learn Examples On Radial Probability Distribution Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com
Solved Interpreting The Radial Protability Distribution O Chegg Com









































































Post a Comment for "Radial Probability Distribution Increasing Energy"